|
 |
| Neurofunction > Volume 17(2); 2021 > Article |
|
Abstract
Meningioma is the most common asymptomatic lesion detected on brain magnetic resonance imaging, and it is usually benign. Because of its generally indolent clinical nature, watchful observation is the preferred primary treatment option in the majority of cases. However, in rare cases, meningioma progresses with exponential growth exceeding the usual expectations, resulting in a very difficult situation for clinical management. Herein, we report a case of small asymptomatic meningioma diagnosed incidentally during the treatment of hemorrhagic stroke. It was left without treatment or regular follow-up and became a huge mass over 7 years. It was then treated with fractionated Gamma Knife radiosurgery and showed significant volume reduction. This case demonstrates the efficacy of fractionated radiosurgery for large meningiomas and simultaneously emphasizes the importance of regular imaging follow-up or proactive treatment upon the initial diagnosis of asymptomatic meningioma, so that we may not miss the optimal timing for treatment.
Meningioma is the most common primary central nervous system tumor, accounting for 37.6% of cases [1]. With the advent of neuroimaging techniques, the prevalence of meningioma is increasing in patients with non-specific neurologic symptoms. More than 80% of meningioma cases are histologically benign World Health Organization grade I tumors that have an indolent natural course. Asymptomatic and small (<2 cm) meningiomas are managed with observation and active surveillance [2]. During serial clinical follow-up, most meningiomas become larger with time, and nearly one-third of patients eventually require surgical resection or stereotactic radiosurgery [3].
The treatment options for intracranial meningioma include surgical resection, stereotactic radiosurgery, and watchful observation; the choice is made considering the patient’s medical history and symptoms, the tumor’s location, and the risk/benefit ratio. In recent studies, primary Gamma Knife radiosurgery (GKS) has shown long-lasting tumor control outcomes compared with Simpson grade I surgical resection for meningiomas <3.5 cm [4,5]. However, with single-fraction stereotactic radiosurgery, a large tumor volume is associated with poorer local control and a higher radiation-related complication rate [6]. The most appropriate patient selection, target volume, radiation dose, and fractionation schedule have not yet been studied in prospective trials [7].
In this report, we describe a case of a rapidly growing, very large meningioma that showed a radiologic response after GKS.
A 58-year-old woman was incidentally diagnosed with a small meningioma located in the left anterior clinoid process (1.1×1.1×1.1 cm3; Fig. 1A) during treatment for hypertensive intracerebral hemorrhage, which caused permanent left-side hemiplegia. The meningioma was not treated owing to her poor general condition and slow tumor growth for the first 16 months on radiologic follow-up (2.0×1.8×2.0 cm3; Fig. 1B). The tumor grew rapidly during a 67-month period after the last follow-up (4.7×4.7×5.0 cm3; Fig. 1C); however, the patient complained of no tumor-related symptoms.
Considering the patient’s impaired self-awareness and dependency on activities of daily living, as well as the risk of surgical intervention and the patient’s and caregivers’ preferences, she underwent fractionated GKS (5 Gy at the 50% isodose line, 4 fractions, prescription isodose volume of 58.6 cm3) with no treatment-related adverse events. The tumor size had decreased at 3 years after radiosurgery, with no radiation-induced changes in adjacent brain tissue (4.2×4.2×4.5 cm3; Fig. 1C). She complained of neither tumor-related symptoms nor treatment-related complications.
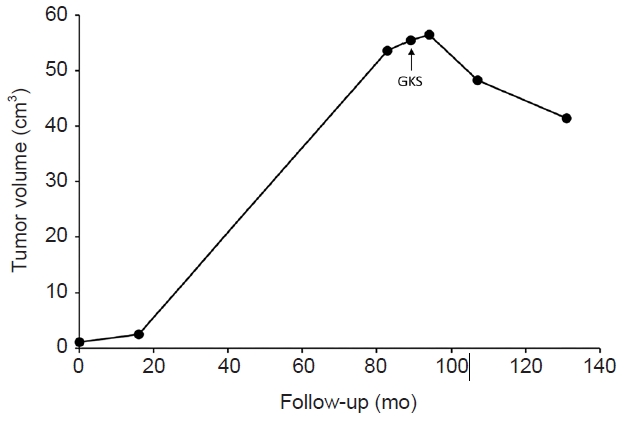
Meningioma is expected to grow in three phases: pre-exponential growth (lag phase), exponential growth, and post-exponential growth (plateau phase) [8]. The meningioma growth pattern changes according to volume expansion. In our case, during an 83-month period, the asymptomatic meningioma grew from 1.1 to 53.6 cm3. The tumor growth rate during the initial 16-month follow-up period was 1.1 cm3/year (lag phase). During the period when the patient was lost to follow-up, the tumor growth rate was 9.2 cm3/year (exponential growth phase). The tumor growth rate in the last 9 months before GKS was 3.2 cm3/year (plateau phase) (Fig. 2). As many asymptomatic meningiomas pass the inflection point and display a self-limiting growth pattern, the overall consensus is that observation with serial imaging follow-up is safe [9]. Several studies have revealed that the appearance of calcification and radiocarbon retrospective birth dating analysis help to predict the growth pattern and guide clinical decisions [8,10].
In a retrospective study investigating the volumetric growth rate of meningioma, the growth rates of grade I and II meningiomas were 0.07-3.26 cm3/year and 3.61-13.29 cm3/year, respectively. A volumetric growth rate of 3.05 cm3/year was suggested as a threshold for grade II meningioma [11]. Another large-cohort retrospective study on the natural history of meningioma that used volumetric assessments suggested a cutoff point of 2 cm3/year as a standard for rapid growth and designed a scoring system, the Asan Intracranial Meningioma Scoring System (AIMSS), for rapid meningioma growth based on a patient’s individual factors [12]. According to AIMSS, our case would receive an intermediate-risk score of 4, which is expected to show a 10-50% risk of rapid tumor growth (≥2 cm3/year). However, in our case, during a total of 7.8 years of follow-up, the tumor volume increased by about 53.6 cm3, corresponding to a growth rate of 6.87 cm3/year, and eventually required an intervention. Therefore, regardless of the rapid tumor growth risk at the time of initial diagnosis, if treatment is not performed immediately, regular follow-up is the only alternative.
Observation is an appropriate choice for asymptomatic small meningiomas (less than 2.5 cm in size) [13]. Annual or biennial follow-up with serial imaging is recommended [9]. During the follow-up period, the risk of accelerated growth and progression with a tendency for malignancy and parenchymal invasion should be considered.
GKS has proven to be a viable option for achieving tumor control in the management of meningioma and also has become a first-line treatment for some meningiomas, especially skull base lesions involving the petroclival region, the cavernous sinus, or cranial nerves [14]. As GKS delivers localized irradiation, a lower volume of the surrounding normal tissue is exposed to radiation, resulting in reduced normal brain toxicity. GKS for meningioma has traditionally been performed in a single session; however, reports of hypofractionated GKS are emerging [15,16]. Fractionated GKS provides an improved balance of tumor control and adjacent normal brain toxicity. Unlike several studies reporting poor outcomes of single fraction radiosurgery for large meningiomas [6,17], we could achieve a satisfactory result using fractionated GKS for this large, rapidly growing meningioma located near critical neurovascular structures.
Fig. 1.
Contrast-enhanced axial T1 magnetic resonance imaging (MRI) (A) shows a small meningioma in the left anterior clinoid process. With serial follow-up, MRI scans (B) showed tumor progression. The patient underwent fractionated Gamma Knife radiosurgery (GKS) for the lesion in 4 sessions. During follow-up after GKS, MRI scans (C) showed that tumor regression was achieved. No new symptoms developed during the follow-up period.
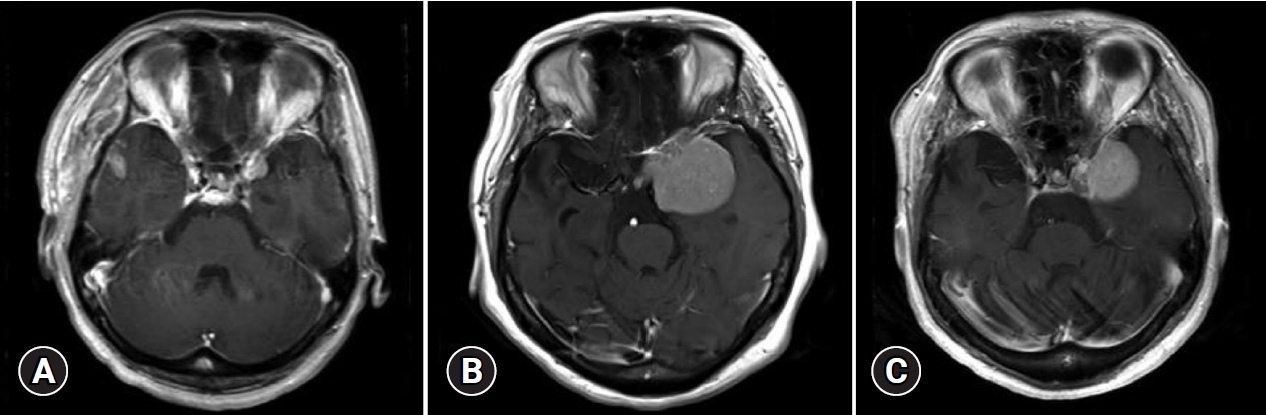
Fig. 2.
Volumetric changes over time during observation and after Gamma Knife radiosurgery (GKS). The tumor growth rate during the initial 16-month follow-up period was 1.1 cm3/year. During the period when the patient was lost to follow-up, the tumor growth rate was 9.2 cm3/year. The tumor growth rate in the last 9 months before GKS was 3.2 cm3/year.
REFERENCES
1. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol 2019;21:v1-100



2. Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 2016;17:e383-9


3. Kim KH, Kang SJ, Choi J, Kong D, Seol HJ, Nam D, et al. Clinical and radiological outcomes of proactive Gamma Knife surgery for asymptomatic meningiomas compared with the natural course without intervention. J Neurosurg 2019;130:1740-9

4. Kondziolka D, Patel AD, Kano H, Flickinger JC, Lunsford LD. Long-term outcomes after gamma knife radiosurgery for meningiomas. Am J Clin Oncol 2016;39:453-7


5. Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys 2003;55:1000-5


6. Kirkpatrick JP, Soltys SG, Lo SS, Beal K, Shrieve DC, Brown PD. The radiosurgery fractionation quandary: single fraction or hypofractionation? Neuro Oncol 2017;19:ii38-49



7. Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg 2015;122:4-23



8. Huttner HB, Bergmann O, Salehpour M, El Cheikh R, Nakamura M, Tortora A, et al. Meningioma growth dynamics assessed by radiocarbon retrospective birth dating. EBioMedicine 2018;27:176-81


9. Behbahani M, Skeie GO, Eide GE, Hausken A, Lund-Johansen M, Skeie BS. A prospective study of the natural history of incidental meningioma-Hold your horses! Neurooncol Pract 2019;6:438-50



10. Nakasu S, Fukami T, Nakajima M, Watanabe K, Ichikawa M, Matsuda M. Growth pattern changes of meningiomas: long-term analysis. Neurosurgery 2005;56:946-55. discussion 946-55


11. Soon WC, Fountain DM, Koczyk K, Abdulla M, Giri S, Allinson K, et al. Correlation of volumetric growth and histological grade in 50 meningiomas. Acta Neurochir (Wien) 2017;159:2169-77


12. Lee EJ, Kim JH, Park ES, Kim YH, Lee JK, Hong SH, et al. A novel weighted scoring system for estimating the risk of rapid growth in untreated intracranial meningiomas. J Neurosurg 2017;127:971-80


13. Lee EJ, Park JH, Park ES, Kim JH. “Wait-and-See” strategies for newly diagnosed intracranial meningiomas based on the risk of future observation failure. World Neurosurg 2017;107:604-11


14. Prabhu VC, Melian E, Germanwala AV, Solanki AA, Borys E, Barton K, et al. Cranial base meningiomas. World Neurosurg 2018;109:258-62


15. Morimoto M, Yoshioka Y, Shiomi H, Isohashi F, Konishi K, Kotsuma T, et al. Significance of tumor volume related to peritumoral edema in intracranial meningioma treated with extreme hypofractionated stereotactic radiation therapy in three to five fractions. Jpn J Clin Oncol 2011;41:609-16


-
METRICS

-
- 0 Crossref
- 0 Scopus
- 2,193 View
- 7 Download
- ORCID iDs
-
Jung-Il Lee

https://orcid.org/0000-0001-8143-5513 - Related articles
-
Cyst Associated with Meningioma Treated Using Gamma Knife Radiosurgery2017 June;13(1)
Metastatic Cardiac Fibrosarcoma Managed with Gamma Knife Radiosurgery (GKRS)2008 June;4(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print