|
 |
| Neurofunction > Volume 20(1); 2024 > Article |
|
Abstract
Progressive multifocal leukoencephalopathy (PML) is a rare demyelinating disease of the central nervous system caused by the John Cunningham virus (JCV). It typically occurs in patients with advanced HIV infection. We report the case of a 31-year-old male patient who presented with symptoms of dizziness, dysarthria, ataxia, and diplopia, and was subsequently diagnosed with HIV/AIDS. Despite the negative JCV-polymerase chain reaction (PCR) results on cerebrospinal fluid (CSF) analysis, brain magnetic resonance imaging revealed characteristic features of PML. Therefore, a brain biopsy was performed, which revealed lymphohistiocytic infiltration and perivascular lymphoid cuffing, along with positive immunohistochemical staining for SV40, a JC viral protein. After the diagnosis of PML, the patient developed immune reconstitution inflammatory syndrome, and corticosteroids were administered. Our case emphasizes that negative JCV-PCR results on CSF analysis do not necessarily rule out the diagnosis of PML. Clinicians should consider brain biopsy as a diagnostic tool for PML, even when CSF JCV-PCR results are negative. An early brain biopsy may be necessary to provide proper management to patients with PML.
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disorder of the central nervous system caused by the John Cunningham virus (JCV). Depending on the affected area, PML can manifest with various focal neurological abnormalities such as motor dysfunction, cognitive impairment, visual deficits, and dementia. PML primarily affects individuals with compromised immune function, including those with AIDS, lymphoma, sarcoidosis, or receiving long-term immunosuppressive therapy [1]. The diagnosis of PML requires confirmation of the presence of JCV DNA by polymerase chain reaction (PCR) analysis of cerebrospinal fluid (CSF) [2].
However, the sensitivity of CSF JCV-PCR has decreased to 57.5% due to viral clearance resulting from immune reconstitution in HIV/AIDS patients treated with highly active antiretroviral therapy (HAART) [3]. Consequently, in cases where clinical and radiological suspicion of PML persists despite negative CSF JCV-PCR results, a brain biopsy should be considered for definitive diagnosis. Herein, we present a case of PML diagnosed through stereotactic brain biopsy in a patient with negative JCV DNA PCR result.
A 31-year-old male with no significant medical history presented with dizziness, dysarthria, motor weakness, and blurred vision that had started 2 months prior to admission. His symptoms worsened over the past month, with gait instability and falls during walking.
Upon admission, neurological examination revealed left-beating nystagmus on left gaze. Mild ataxia was observed on the finger-to-nose and heel-to-shin tests, and instability was seen on the tandem gait test. Complete blood count revealed leukopenia with a white blood cell count of 1,700/μL (56.8% neutrophils), hemoglobin of 14.5 g/dL, and platelets of 133,000/μL. Serologic testing revealed HIV positivity, leading to an AIDS diagnosis.
Brain magnetic resonance imaging (MRI) showed mild left cerebellar and left middle cerebellar peduncle swelling with T2 hyperintense lesions. Small T2 hyperintense lesions were also observed in the right thalamus, periventricular white matter of the left lateral ventricle, and frontal subcortical white matter of the left frontal lobe, without contrast enhancement (Fig. 1A).
These findings suggested a demyelinating disorder, particularly PML. Therefore, CSF JCV DNA PCR was performed but yielded a negative result.
Subsequently, the patient’s dysarthria, gait disturbances, and dizziness worsened, and new symptoms of left-sided weakness and right-sided ataxia developed. Despite symptomatic treatment and antiretroviral therapy (ART) for PML, the patient’s symptoms did not improve and continued to deteriorate. Considering the possibility of HIV encephalopathy, primary central nervous system (CNS) lymphoma, cerebral toxoplasmosis, or other infectious diseases that can occur in AIDS patients, a diagnostic workup was done, leading to a stereotactic brain biopsy in the region of interest.
A frame-based stereotactic needle aspiration biopsy was performed. The patient’s head was immobilized using a Leksell stereotactic frame G (Elekta Instruments AB), and MRI was taken to identify the target. The MRI data, acquired with the frame fixed, were subsequently registered in Surgiplan software (Elekta AB; Elekta Company) to devise a trajectory. Six specimens were achieved from the patient’s brainstem lesion.
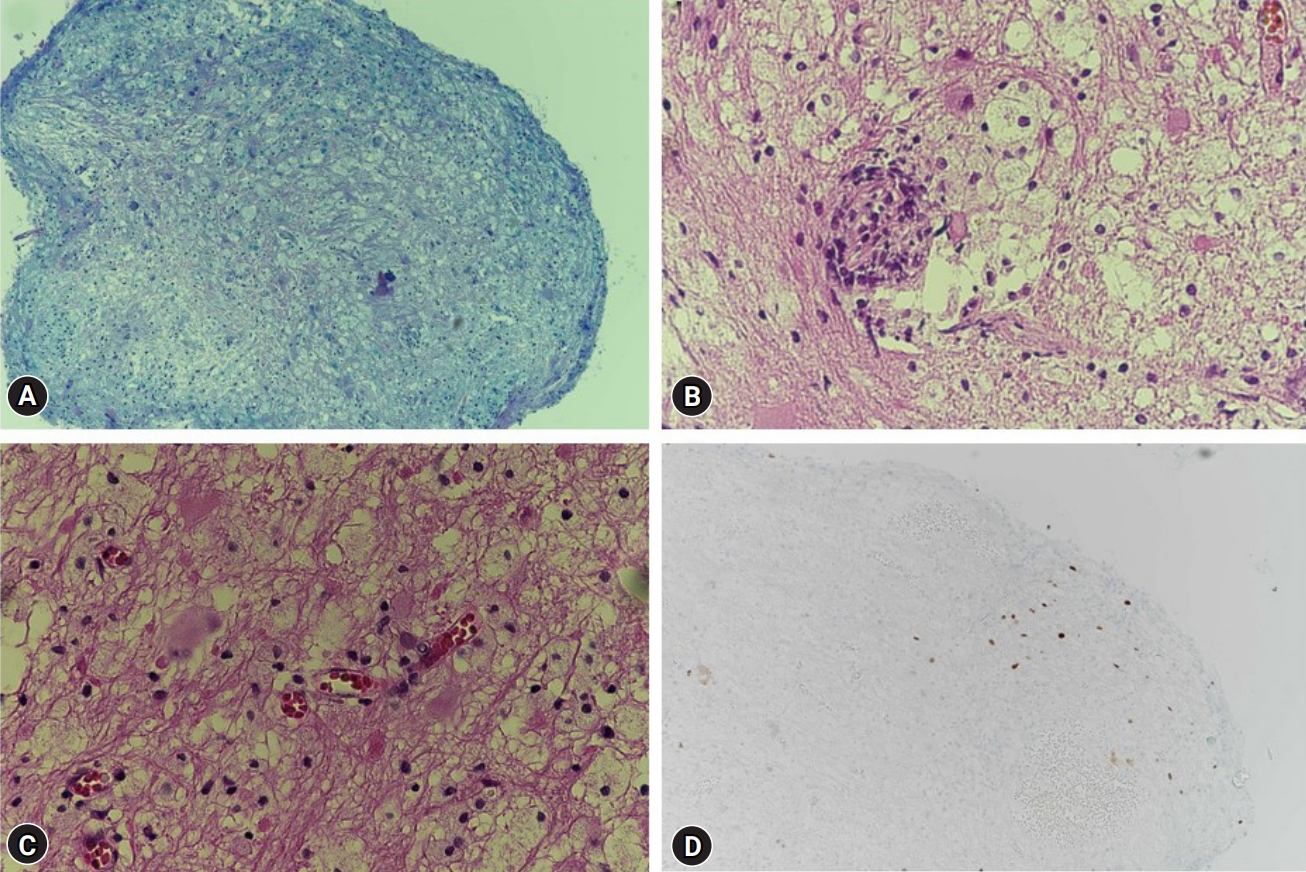
Histopathological examination of the biopsy specimen revealed lymphocyte and macrophage infiltration. Immunohistochemistry staining for SV40, a JCV protein, demonstrated the presence of SV40-positive cells forming viral inclusions, confirming the diagnosis of PML (Fig. 2).
The patient was treated with bictegravir/emtricitabine/tenofovir alafenamide as part of ART. Follow-up brain MRI showed peripheral enhancement around the T2 hyperintense lesion in the subcortical white matter of the left frontal lobe (Fig. 1B), indicating the development of immune reconstitution inflammatory syndrome (IRIS). To manage this, oral dexamethasone (60 mg) was administered. The patient’s symptoms improved after comprehensive rehabilitation, and subsequent brain MRI showed a reduction in the lesions (Fig. 1C).
PML is an inflammatory demyelinating disease that occurs in multiple areas of the brain’s white matter, caused by the JCV. PML predominantly occurs in individuals with severe immunosuppression, such as those with HIV/AIDS, and accounts for 2-14% of neurologic disorders associated with HIV/AIDS. Clinical symptoms of PML manifest as various neurological manifestations depending on the location of the lesions, including visual disturbances, weakness, cognitive impairment, speech disorders, sensory deficits, and cerebellar dysfunction. Brain MRI shows multifocal, non-enhancing, and hypointense lesions on T1-weighted images and hyperintense lesions on T2-weighted images that are widely distributed in the white matter [4]. Therefore, when characteristic multifocal demyelinating lesions are observed on brain MRI in HIV/AIDS patients with neurological abnormalities, PML should be considered.
The radiologic characteristics of PML are that it occurs primarily in the frontal and parieto-occipital areas and subcortical U fibers in these areas [5]. Cases of involvement of the posteior fossa also have been reported, and in this case, the cerebellum and middle cerebellar peduncle are commonly involved [6]. Lesions have sharp borders toward gray matter contrasting with ill-defined borders toward white matter in T2-weighted and diffusion weighted image [7]. A T2-weighted image may show a microcyst or granular pattern. Usually, the enhancement is not clear in gadolinium enhanced images, but in some patients, hazy peripheral enhancement may occur. Diagnoses that need to be differentiated radiologically include multiple sclerosis, posterior reversible encephalopathy syndrome, acute disseminated encephalomyelitis, and primary CNS lymphoma [8].
According to the diagnostic guidelines proposed by the American Association of Neurology in 2013, CSF JCV-PCR testing is conducted when PML is suspected based on clinical symptoms and radiological findings. In cases where the initial CSF JCV-PCR test yields a negative result, repeating the test is recommended. If the PCR test continues to yield negative results, a brain biopsy is advised.
However, the value of repeating the PCR test when the initial CSF JCV-PCR test is negative remains uncertain. Several cases have been reported where PML was confirmed by brain biopsy despite initially negative CSF JCV-PCR test results [9-11]. Additionally, a case was documented where repeated CSF JCV-PCR testing performed three times yielded negative results, but subsequent brain biopsy confirmed PML [12]. A study demonstrated that the sensitivity of CSF JCV-PCR has decreased to 57.5% due to decreased JCV DNA titers in CSF after the introduction of HAART in HIV/AIDS patients [13]. In our case, despite strong suspicion of PML based on clinical symptoms and radiological findings, the CSF JCV-PCR test yielded a negative result.
Therefore, considering the high false-negative rate of JCV-PCR testing, early brain biopsy should be considered when the initial JCV-PCR test is negative. Brain biopsy is an invasive procedure with a sensitivity of 93-96% and specificity of 100% for detecting localized brain lesions in HIV/AIDS patients.
In our case, a stereotactic brain biopsy was performed. Although brainstem biopsy carries potential risks of neurologic sequalae, biopsy of the brainstem was conducted as the initial lesion suspected to be the origin of PML was located there, with a high diagnostic yield expected. The biopsied specimen exhibited infiltration of lymphocytes and macrophages, and immunohistochemical staining for SV40 demonstrated the presence of SV40-positive cells forming viral inclusion bodies, confirming the diagnosis of PML.
The 1-year survival rate for HIV+/PML patients is approximately 52%, while it is around 58% for HIV-/PML patients [10]. Studies have suggested that ART improves the average survival period in HIV+/PML patients, and the effects of mefloquine and mirtazapine therapy have been reported in HIV-/PML patients [14-16]. Therefore, early diagnosis of PML and appropriate treatment may potentially improve the patient’s survival rate. Particularly in cases where the disease progresses to PML-IRIS, steroid therapy can be considered; however, since the use of steroids can induce immunosuppression, it is crucial to exclude other infectious conditions through differential diagnosis before starting steroid treatment. In our case, the disease progressed to PML-IRIS, and steroid therapy was initiated 5 days after the brain biopsy. By confirming PML through brain biopsy and ruling out other infectious diseases, steroid therapy was initiated. Thus, in situations where clinical suspicion of PML remains despite a negative CSF JCV-PCR result, early brain biopsy can aid in treatment decisions for PML-IRIS patients.
We presented a case of PML in a patient with negative JCV-PCR results on CSF analysis. The negative PCR results did not rule out the diagnosis of PML, and a brain biopsy was essential for confirming the diagnosis. Clinicians should consider brain biopsy as a diagnostic tool for PML, even when CSF JCV-PCR results are negative. Early brain biopsy may be necessary to provide proper management for patients with PML. Further studies are warranted to explore the role of brain biopsy in cases of suspected PML with negative CSF JCV-PCR results.
Fig. 1.
Serial magnetic resonance image findings on fluid-attenuated inversion recovery images. (A) On admission, hyperintense lesions were seen in the left cerebellar peduncle, left periventricular white matter, and left frontal subcortical white matter. (B) After a brain biopsy, a newly appearing hyperintense lesion in the subcortical white matter of the left frontal lobe was noted. (C) After corticosteroid treatment, the progressive multifocal leukoencephalopathy lesions resolved.

Fig. 2.
Histopathologic examination. (A) Demyelinated lesions (Luxol fast blue, ×100). (B) Lymphohistiocytic infiltration and perivascular lymphoid cuffing (hematoxylin and eosin [H&E] stain, ×400). (C) Bizarre astrocytes and enlarged oligodendrocytic nuclei (H&E stain, ×400). (D) Some simian virus 40 (SV40)-positive cells showing viral inclusions (SV40, ×100).
REFERENCES
1. Greenlee JE. Progressive multifocal leukoencephalopathy: progress made and lessons relearned. N Engl J Med 1998;338:1378-80


2. Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013;80:1430-8



3. Marzocchetti A, Di Giambenedetto S, Cingolani A, Ammassari A, Cauda R, De Luca A. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol 2005;43:4175-7



5. Yousry TA, Pelletier D, Cadavid D, Gass A, Richert ND, Radue EW, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2012;72:779-87


6. Scholten P, Kralt P, Jacobs B. Posterior fossa progressive multifocal leukoencephalopathy: first presentation of an unknown autoimmune disease. BMJ Case Rep 2017;2017:bcr2017220990



7. Hodel J, Outteryck O, Dubron C, Dutouquet B, Benadjaoud MA, Duhin E, et al. Asymptomatic progressive multifocal leukoencephalopathy associated with natalizumab: diagnostic precision with MR imaging. Radiology 2016;278:863-72


8. Kartau M, Sipilä JO, Auvinen E, Palomäki M, Verkkoniemi-Ahola A. Progressive multifocal leukoencephalopathy: current insights. Degener Neurol Neuromuscul Dis 2019;9:109-21



9. Landry ML, Eid T, Bannykh S, Major E. False negative PCR despite high levels of JC virus DNA in spinal fluid: implications for diagnostic testing. J Clin Virol 2008;43:247-9



10. Kuhle J, Gosert R, Bühler R, Derfuss T, Sutter R, Yaldizli O, et al. Management and outcome of CSF-JC virus PCR-negative PML in a natalizumab-treated patient with MS. Neurology 2011;77:2010-6



11. Ikeda J, Matsushima A, Ishii W, Goto T, Takahashi K, Nakamichi K, et al. Brain biopsy is more reliable than the DNA test for JC virus in cerebrospinal fluid for the diagnosis of progressive multifocal leukoencephalopathy. Intern Med 2017;56:1231-4



12. Wang M, Zhang Z, Shi J, Liu H, Zhang B, Yan J. Progressive multifocal leukoencephalopathy in an HIV patient was diagnosed by 3 times lumbar punctures and 2 times brain biopsies. J Neurovirol 2020;26:952-6



13. Falcó V, Olmo M, del Saz SV, Guelar A, Santos JR, Gutiérrez M, et al. Influence of HAART on the clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicenter study. J Acquir Immune Defic Syndr 2008;49:26-31


14. Verma S, Cikurel K, Koralnik IJ, Morgello S, Cunningham-Rundles C, Weinstein ZR, et al. Mirtazapine in progressive multifocal leukoencephalopathy associated with polycythemia vera. J Infect Dis 2007;196:709-11


-
METRICS

-
- 0 Crossref
- 0 Scopus
- 338 View
- 8 Download
- ORCID iDs
-
Seung Woo Hong

https://orcid.org/0000-0003-3021-5216 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print